Abstract
Introduction
Myelodysplastic syndrome (MDS) is mainly a disease of the elderly, with a median age of 72 years. There is little information regarding Adolescent and Young Adult (AYA) Patients with Myelodysplastic syndrome (MDS). AYA cancer patients are defined as those patients ages between 15-39 years according to NCCN guidelines. This retrospective study describes the general characteristics, cytogenetics, mutational profiles, treatments, and outcomes of AYA with MDS Diagnosis.
Methodology
We analyzed the clinical database of a single tertiary care center for patients with MDS ages between 18- 39 years from January 2012 through December 2020. We used 18 years as age cut-off, and not 15, due to the structure of our cancer center.
Results
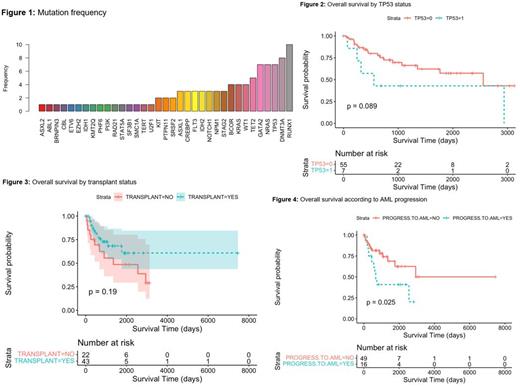
In this retrospective study, 65 patients were identified. The median age was 30 (18-39) years, with female sex predominance (n=37) (57%). Baseline laboratory findings: median hemoglobin (HgB) was 9.45gm/dL (6.2-14.8), white blood cells (WBC) (3.4x10e9/L [0.3-136.9], platelets 63,000 (5,000-479,000), bone marrow blast 4% [0-17], IPSS was low in 11 patients (17%), intermediate- 1 in 23 (35%), intermediate- 2 16(25%) and high 8 (12%). 58 patients (89%) had MDS and seven (11%) had CMML. Twenty patients (30.7%) had a previous history of other cancers, with sarcomas being the most frequent with 6 cases (9.2%). Therapy-related MDS (t-MDS) was observed in 18 patients (27.6%). Ten patients (15.3%) had bone marrow failure syndrome, with GATA2 syndrome being the most frequent. Fanconi anemia and Schwachman-Diamond Syndrome was documented in two patients respectively. The most recurrent cytogenetics alterations were diploid in 20 patients (30.7%), followed by complex in 11 (16.9%). The most frequent mutations were RUNX1 (somatic)15%, followed by DNMT3A, TP53, NRAS, GATA2 and TET2, as shown in the Figure 1.
Hypomethylating agents (HMAs) were the most frequent first line treatment used in 16 patients (24.6%). Forty-three patients (66%) underwent an allogeneic bone marrow transplant with a median OS (95% CI) of 27 months (9-45). While for the group of patients who didn't receive transplant, it was 21 months (7-69) (p=0.19) vs patients who didn't receive transplant. Allogeneic transplantation in TP53-mutated patients resulted in a Median OS (95% CI) of 21 months (12-65). Patients who progressed into AML had an inferior median OS (95% CI) of 21 months (12-65) for vs 28 months (11-47) for those who did not progressed to AML(p=0.025). In multivariate analysis expression of RUNX1 and NOTCH1, was associated with inferior outcomes (p-value=0.035, 0.004 respectively) (Figure 2,3 and 4)
Conclusion
In our cohort, MDS occurred as part of marrow failure syndrome or consequence of therapy t-MDS. Somatic RUNX1 was the most frequent mutation in AYA group with MDS. RUNX1, NOTCH1 and Tp53 mutated patients had worse outcome. Most patients underwent bone marrow transplant
Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Daver: Abbvie: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Glycomimetics: Research Funding; Novimmune: Research Funding; Amgen: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Hanmi: Research Funding; Sevier: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Kadia: Liberum: Consultancy; Novartis: Consultancy; Pfizer: Consultancy, Other; Jazz: Consultancy; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Pulmotech: Other; Genentech: Consultancy, Other: Grant/research support; Aglos: Consultancy; Sanofi-Aventis: Consultancy; Genfleet: Other; Astellas: Other; Ascentage: Other; AstraZeneca: Other; AbbVie: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Cure: Speakers Bureau; Cellonkos: Other. Pemmaraju: LFB Biotechnologies: Consultancy; Incyte: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; CareDx, Inc.: Consultancy; DAVA Oncology: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Cellectis S.A. ADR: Other, Research Funding; Celgene Corporation: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Roche Diagnostics: Consultancy; Daiichi Sankyo, Inc.: Other, Research Funding; Affymetrix: Consultancy, Research Funding; Plexxicon: Other, Research Funding; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Consultancy; Springer Science + Business Media: Other; MustangBio: Consultancy, Other; Sager Strong Foundation: Other; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Kantarjian: NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria; Precision Biosciences: Honoraria; BMS: Research Funding; Amgen: Honoraria, Research Funding; Jazz: Research Funding; Ascentage: Research Funding; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; Ipsen Pharmaceuticals: Honoraria; Astra Zeneca: Honoraria; Astellas Health: Honoraria; Aptitude Health: Honoraria; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Taiho Pharmaceutical Canada: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal